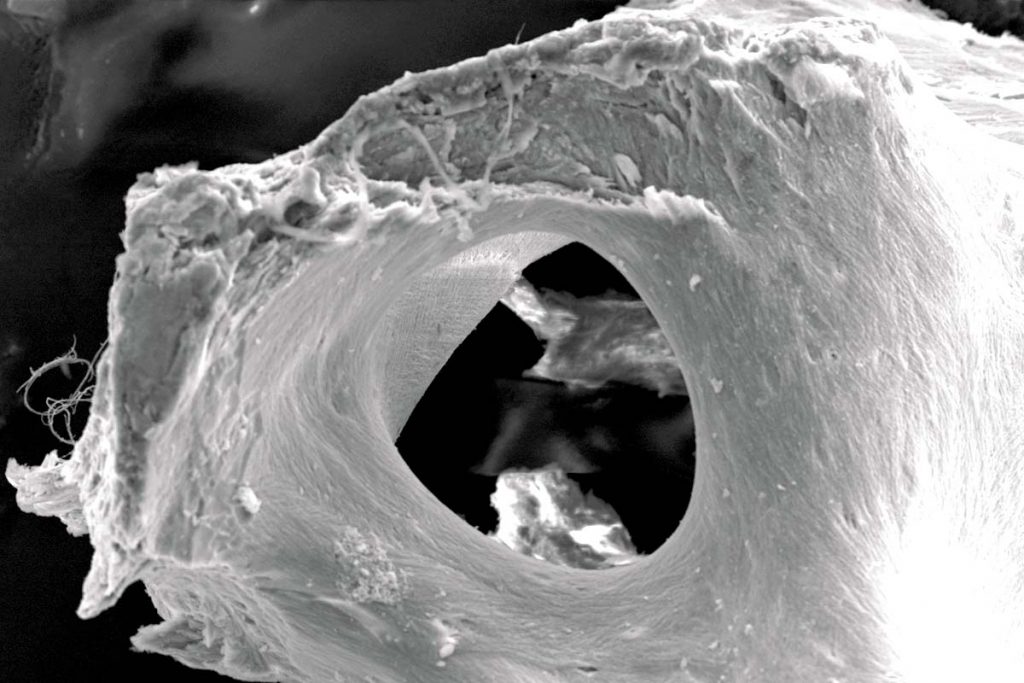
maxgraft® bonebuilder
Customized allogenic bone block
maxgraft® bonebuilder is a customized allogenic bone block for two-stage alveolar ridge augmentation. Based on CT/CBCT scans of the patient, the bone block is virtually designed using the latest 3D-CAD/CAM (computer-aided design/computer-aided manufacturing) technology. The final product is then milled from processed cancellous bone blocks originating from living human donors (explantation of femoral heads during hip endoprosthesis surgery).
In clinical practice, the application of allogenic blocks has been established as a reliable alternative to autogenous bone blocks for alveolar ridge augmentation to avoid donor site morbidity1,2,3 and limitations in material quantity4.
maxgraft® bonebuilder offers greater precision and accuracy of fit compared to classical block augmentation. Manual adjustment of the block during the operation is seldom required and maxgraft® bonebuilder may be applied directly onto the defect, reducing surgery time as well as risk of infection.5,6 The individual design provides a precise fit between local bone and the allogenic bone block, enabling rapid revascularization and fast graft incorporation.7
Achieve a safe, reliable and predictable clinical outcome
Due to the stable trabecular structure of the cancellous bone, maxgraft® bonebuilder provides an ideal matrix for predictable revascularization, rapid formation of new bone tissue and bone remodeling.7 Simultaneously the biological regeneration capability of maxgraft® is supported by good flexibility on the basis of natural collagen content, which will facilitate screw fixation.
The processing sequence meets high quality standards with regard to biomechanical properties and safety. maxgraft® is storable at room temperature (5-30°C) for 5 years. However, the block should be used as soon as possible after delivery to provide a precise fit, enabling rapid revascularization and fast graft incorporation.
INDICATIONS
Implantology, Oral and CMF Surgery
Horizontal and vertical augmentation
Extensive bone defects
Product properties and specifications
maxgraft® bonebuilder
+ Customized, processed allograft (from selected living donors)
+ Mineralized human collagen for excellent biocompatibility and flexibility
+ Stable trabecular structure of the cancellous bone enables rapid revascularization
+ Osteoconductive properties support natural and controlled bone remodeling
+ Max. dimensions 23 x 13 x 13 mm
| Art.-No. | Content | |
|---|---|---|
| PMIa | Individual planning and production of a bone block max. dimensions 23 x 13 x 13 mm | |
| PMIa 2 | maxgraft® bonebuilder, additional block(s) for this patient |
bonebuilder dummy
An individual 3D-model of the patient’s defect, including the planned maxgraft® bonebuilder block(s) printed in synthetic filament, can be ordered together with the maxgraft® bonebuilder and delivered beforehand. The user-friendly demo material helps to inform the patient and supports preoperative planning.
| Art.-No. | Content | |
|---|---|---|
| 32100 | Individual 3D-printed model of the patient’s defect and the planned maxgraft® bonebuilder for demonstration purposes made of plastic |
SPECIFIC FACTS
Support/Contact
Mr. Goran Nikoloski +49 30 20 60 73 98 35
Ms. Gina Grosser +49 30 20 60 73 98 68
Ms. Kristina Domann +49 30 20 60 73 98 26
We offer the printing of individual 3D models.
For an individual offer or support, contact us.
References
1 Monje, A. et al. (2014). On the feasibility of utilizing allogeneic bone blocks for atrophic maxillary augmentation. Biomed Res. Int. 2014, 814578.
2 Novell, J. et al. (2012). Five-year results of implants inserted into freeze-dried block allografts. Implant Dent. 21, 129–35.
3 Nissan, J. et al. (2011). Cancellous bone block allografts for the augmentation of the anterior atrophic maxilla. Clin. Implant Dent. Relat. Res. 13, 104–11.
4 Jacotti, M. et al.(2012). Ridge augmentation with mineralized block allografts: clinical and histological evaluation of 8 cases treated with the 3-dimensional block technique. Implant Dent. 21, 444–8.
5 Jacotti, M. et al. (2014). Posterior atrophic mandible rehabilitation with onlay allograft created with CAD-CAM procedure: a case report. Implant Dent. 23, 22–8.
6 Blume, O. et al. (2017). Treatment of Severely Resorbed Maxilla Due to Peri-Implantitis by Guided Bone Regeneration Using a Customized Allogenic Bone Block: A Case Report. Materials 10(10): 1213.
7 Kloss FR, et al. (2020). Customized allogeneic bone grafts for maxillary horizontal augmentation: A 5-year follow-up radiographic and histologic evaluation. Clin Case Rep. 2020;00:1–8