Important information regarding delivery times:
Due to the upcoming company vacations 22.12.2025 – 02.01.2026, delivery times will be extended.
You will be informed of the expected delivery date for each order individually.
Thank you for your understanding.
maxgraft® bonebuilder
CUSTOMIZED ALLOGENIC BONE BLOCK FOR TWO-STAGE ALVEOLAR RIDGE AUGMENTATION
Based on CT/CBCT scans of the patient, the bone block is virtually designed using the latest 3D CAD/CAM (computer-aided design/computer-aided manufacturing) technology.
maxgraft® bonebuilder offers greater precision and accuracy of fit compared to classical block augmentation. Manual adjustment of the block during the operation is seldom required and maxgraft® bonebuilder may be applied directly onto the defect, reducing surgery time as well as risk of infection.1-5 The individual design provides a precise fit between local bone and the allogenic bone block, enabling rapid revascularization and fast graft incorporation.6-7
PLANNING & PRODUCTION
maxgraft® bonebuilder is planned in close interaction between clinical users and botiss CAD-designers with multiple feedback loops to ensure an optimal fit. Communication is mainly done via email using the comment section on the specific order web page.
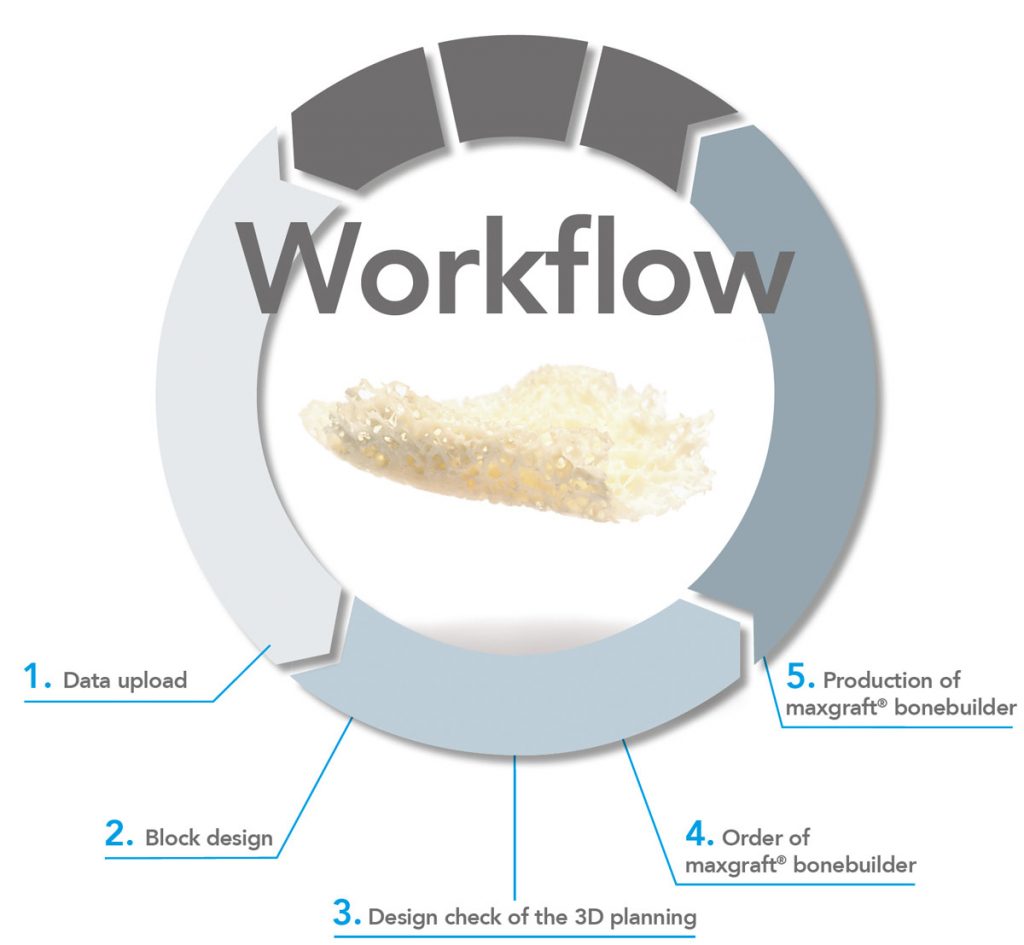
STEP BY STEP
1. Data upload *
2. Block design
3. Design check of the 3D-planning
4. Order of maxgraft® bonebuilder
5. Production
6. Invoice and delivery
* The time between the CBCT scan and the creation of the order should be kept as short as possible. This is because the bone defect may change over time, which could affect the optimal accuracy and overall fit of the block.
THE ORIGIN
maxgraft®
SAFE, RELIABLE AND PREDICTABLE CLINICAL OUTCOME
maxgraft® bonebuilder is produced at the Cells+Tissuebank Austria (C+TBA), a non-profit organization aiming to provide allogenic transplants for orthopedic and dental regeneration situated in Krems/Austria.
maxgraft® bonebuilder is made from cancellous human donor bone and originates from femoral heads (taken during hip surgery) of living donors from certified procurement centers in Europe.
PROPERTIES
+ Processed human allograft from selected living donors
(Production: C+TBA, Krems, Austria)
+ Natural mineralized collagen
+ Maximum size: 23 x 13 x 13 mm
+ Fast graft incorporation and complete remodeling potential
+ No donor site morbidity
+ 5-6 months healing-/integration time
+ 5 years shelf life at 5-30°C
References
1 Jacotti et al. 2014. Posterior atrophic mandible rehabilitation with onlay allograft created with CAD-CAM procedure: a case report. Implant Dent. Feb;23(1):22-8
2 Blume et al. 2017. Treatment of Severely Resorbed Maxilla Due to Peri-Implantitis by Guided Bone Regeneration Using a Customized Allogenic Bone Block: A Case Report. Materials, 10, (10) 1213
3 Blume et al. 2019. Bilateral maxillary augmentation using CAD/CAM manufactured allogenic bone blocks for restoration of congenitally missing teeth: A case report. J Esthet Restor Dent.31(3):171-178
4 Blume et al. 2019. Reconstruction of a Unilateral Alveolar Cleft Using a Customized Allogenic Bone Block and Subsequent Dental Implant Placement in an Adult Patient. J Oral Maxillofac Surg. 77(10):2127.e1-2127.e11
5 Otto et al. 2017. Custom-milled individual allogeneic bone grafts for alveolar cleft osteoplasty—A technical note J Craniomaxillofac Surg. Dec;45(12):1955-1961
6 Kloss et al. 2020. Customized allogeneic bone grafts for maxillary horizontal augmentation: A 5-year follow-up radiographic and histologic evaluation. Clin Case Rep. 2020;00:1–8
7 Tuna et al. 2021. From a CAD/CAM-milled, allogeneic bone block to an implant-supported fixed partial denture with angulated screw channel: a case report. Quintessence Int. 2021;52(1):56-63